Newer techniques that easily and accurately guide breeding of plants and animals, and help treat or prevent diseases, are revolutionizing genetic engineering. They are not only easier, faster and more cost-effective, but also may radically alter how governments define and regulate genetic engineering in medicine and in agriculture.
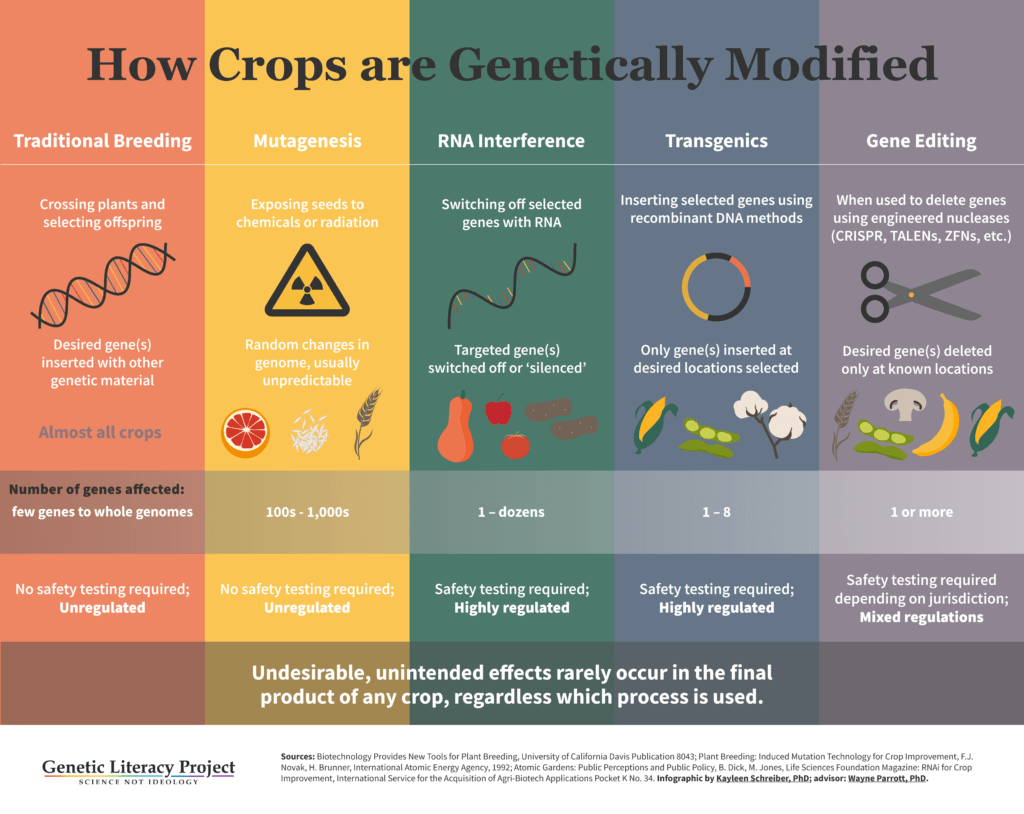
Most genetic engineering techniques have relied on transgenics, modifications made by adding genetic material from different species. These modifications (popularly though inaccurately called “GMO”) have led to successful products, including crops resistant to destructive insects (Bt corn, soy and cotton), and to herbicides (glyphosate, dicamba). But the regulatory process worldwide for these traditional GMOs is arduous and expensive—requiring seven to 13 years of testing and analysis and costing more than one hundred million dollars.
 Anti-biotechnology activists for decades have labeled these crops “Frankenfoods” (the word apparently came from a Boston College professor’s 1992 op-ed in the New York Times). The term stuck because the crops were created by moving so-called “foreign genes” from one species to another. “Plants and organisms unable to [physically] reproduce can become unnaturally intertwined,” reads a typical anti-GMO non-governmental organization (NGO) posting. “A novel gene maybe cobbled together from a plant virus, a soil bacterium and a petunia plant, for example, creating a kind of botanical Frankenstein.”
Anti-biotechnology activists for decades have labeled these crops “Frankenfoods” (the word apparently came from a Boston College professor’s 1992 op-ed in the New York Times). The term stuck because the crops were created by moving so-called “foreign genes” from one species to another. “Plants and organisms unable to [physically] reproduce can become unnaturally intertwined,” reads a typical anti-GMO non-governmental organization (NGO) posting. “A novel gene maybe cobbled together from a plant virus, a soil bacterium and a petunia plant, for example, creating a kind of botanical Frankenstein.”
While these objections overlook the fact that different species of organisms share the same genetic code (DNA), most new breeding techniques (NBTs) don’t move genes between species, rendering the activist complaints irrelevant. Moreover, most new products can be developed in a fraction of the time and at a far lower cost than conventional or transgenic products. The list of NBTs is also not finite—a few have already been abandoned in favor of others, and still more are under development. Currently, NBTs fall into seven broad, scientific categories. The most popular for agricultural biotechnology are CRISPR systems and TALENS. Others include RNA interference (RNAi) and epigenetic techniques.
Gene or genome editing is a broad category in itself that includes techniques that allow scientists to precisely edit genetic material. CRISPR falls into this category. There are three gene editing techniques in existence:
ZFN (zinc finger nuclease)—this is the oldest of the still-used gene-editing techniques, developed in the 1990s, and owned by Sangamo BioSciences. It was first used in Arabidopsis and tobacco plant models of genetics, as well as for research in human diseases such as HIV/AIDS and hemophilia. It also has been used in cotton, soybean, maize and rice. ZFN-based modifications are very time consuming and laborious, and have a high rate of off-target effects, or unintentional changes, reducing their reliability in food or medicine development .
TALENS (Transcription Activator-like Effector Nucleases)—developed in 2009, this technique is more affordable and accurate than ZFN. It has a similar enzymatic, protein engineering structure and function to ZFN. It was used in 2012 to successfully develop disease-resistant rice. It also has been used to create hornless cattle (which do not have to undergo the painful de-horning process now used on dairy farms), higher quality soybean oil, and to add important traits to wheat, potatoes, sugarcane and rice. Like ZFN, TALENS frequently results in “off-target” gene edits and is very time consuming.
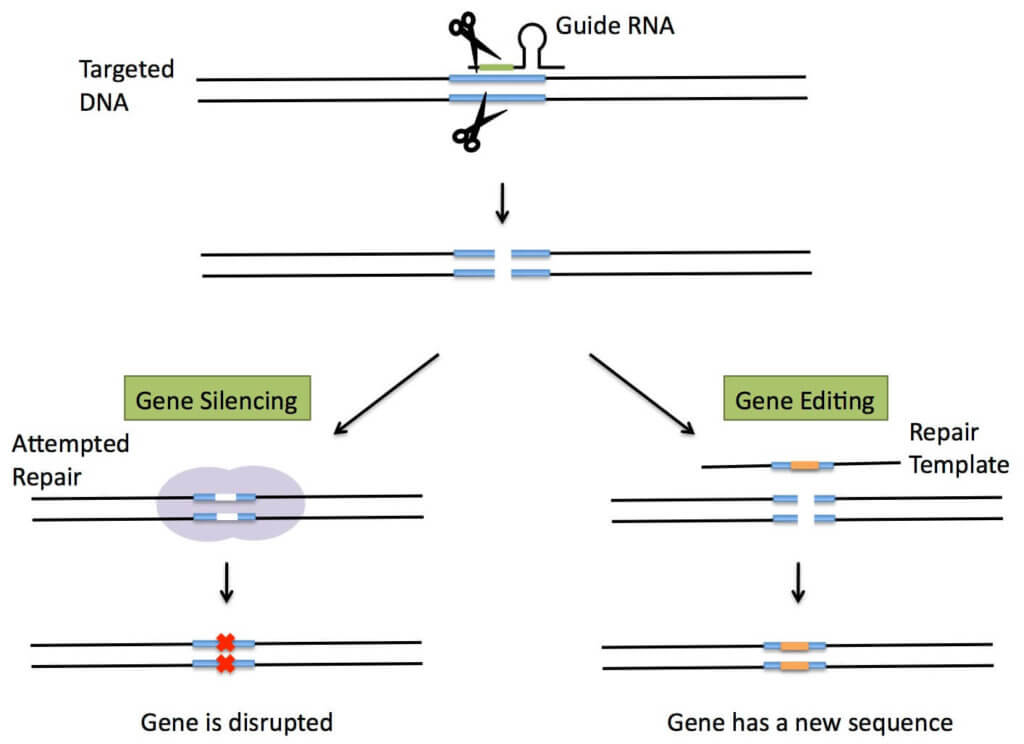
CRISPR-Cas9 (Clustered Regulatory Interspersed Short Palindromic Repeats)—the newest and most powerful of gene editing techniques. Two teams—one led by George Church at Harvard and the Broad Institute, the other by Jennifer Doudna and Emmanuelle Charpentier at UC Berkeley—claim invention of the technique. CRISPR-Cas9 is quite different from ZFN and TALENS; it’s based on a natural bacterial immune system designed to defend cells against invaders by splitting up invasive DNA (or RNA). It is much more affordable, rapid and accurate than other techniques, making it an attractive choice to develop a wide variety of agricultural and biomedical applications.
Scientists were able to reproduce this natural “molecular scissor” technique, in which they cut a section of DNA, and then either bring the loose DNA ends together (eliminating an undesired trait), or insert new DNA expressing a desired trait. In its natural state CRISPR recognizes viral DNA, but scientists have since modified this activity so that CRISPR can cut any DNA at any predetermined location. As the Royal Swedish Academy of Sciences wrote in its Nobel Prize announcement, “Where the DNA is cut it is then easy to rewrite the code of life.”
Human disease advances
In 2019, scientists at Vertex Pharmaceuticals in Boston and CRISPR Therapeutics in Cambridge, Massachusetts, used CRISPR-Cas9 to edit the diseased blood-producing stem cells of a woman suffering from sickle cell disease. After correcting mutations in the HBB gene, which in sickle cell makes misshaped red blood cells, the edited cells were returned, and the woman has been symptom-free since. The companies have since treated dozens more patients with either sickle cell or beta-thalassemia, another genetic blood cell disorder (also caused by HBB mutations).
Scientists also used the technique to edit a gene for a rare form of blindness, this time injecting the gene-editing CRISPR-Cas complex into the patient’s eye, though the long-term efficacy of the procedure is still unknown.
Agricultural advances
Agricultural applications of CRISPR-Cas9 have progressed more rapidly, at least in the United States. The US Department of Agriculture (USDA) revamped its rules in 2019 to allow for more rapid approval (or, in USDA parlance, “deregulation”) of gene-edited plants. In 2019 the USDA approved only seven plants. In 2020, after the reforms took effect, the agency approved more than 70 gene-edited plants, though products that are “pesticidal in nature” are subject to additional oversight from the Environmental Protection Agency (EPA).
The Food and Drug Administration (FDA), which oversees animal biotechnology, takes a far more cautious approach than the USDA, and only one gene-edited animal has been approved in the US. In December 2020, the FDA approved the GalSafe pig, a line of domestic pigs developed by Revivicor. GalSafe pigs were edited, though via an older gene knockout approach, to remove alpha-gal sugars from cell surfaces, reducing the risk of allergic reactions in people with Alpha-gal syndrome. This allows wider use for food consumption as well as organ transplants.
The European Union, which already heavily restricts traditional GMOs, partly because of the movement of genes between species, has determined that gene-editing techniques will be regulated in the same way as GMOs. This July 2018 ruling by the European Court of Justice has made it nearly impossible for developers to commercialize gene-edited crops for cultivation in the EU, though imports are still permitted. Some countries, such as Sweden and France, have disputed the EU’s determination, concluding that CRISPR-edited plants without foreign DNA should not be defined as GMOs. Post-Brexit Great Britain, no longer part of the EU, is also developing its own NBT regulations.
On the other hand, critics of GMOs argue that gene-edited crops should not be regulated any differently than transgenic plants, alleging that unintended edits make NBTs inherently risky and thus subject to heightened regulatory scrutiny. Biotechnology experts have replied that gene editing is far more predictable than conventional crop or animal breeding, which typically results in thousands of unintended mutations and yet poses very low risk to human health.
Other non-gene editing NBTs include:
RNAi (RNA interference) — a natural pathway in the regulation of gene expression, turning genes on and off. It works by attacking the messenger RNA carrying instructions for the targeted genetic trait. RNAi has been used in several crops already, including the approved Arctic Apple and various Simplot Innate Potatoes. Scientists have also used RNAi to develop insect- and disease-resistant crops, crops that neutralize cancer-causing aflatoxin and are exploring its possible use to target bee-killing varroa mites and other harmful pests. Monsanto (now owned by Bayer) is working on an RNAi spray to combat weeds that have developed resistance to its glyphosate herbicide. The spray would neutralize the resistance in those weeds.
Agroinfiltration — used to induce transient gene expression in plants or even in plant cell cultures, and is mostly confined to research or the production of drug proteins.
Epigenetics — these approaches, which do not change but instead regulate a genome (such as RNA-directed DNA methylation) are being explored to manipulate plant DNA without permanently changing it. Modified crops such as soybeans, tomatoes and sorghum have shown increased yields and stress tolerance.
Site-directed mutagenesis (aka oligonucleotide-directed mutagenesis) — a more targeted form of chemical or radiation mutagenesis, two techniques which have been in use since the 1930s and resulted in some 3,000 plants. This technique is now used mostly as an investigative tool.


